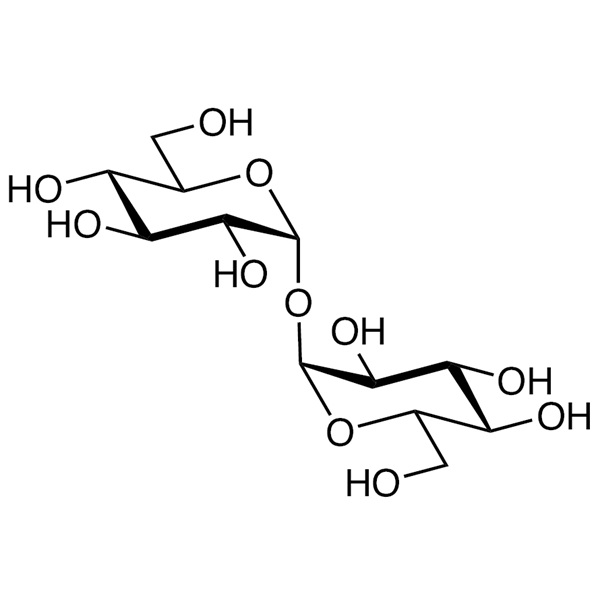
D-(+)-Trehalose Anhydrous CAS 99-20-7 Assay >99.0% (HPLC) Factory
Ruifu Chemical is the leading manufacturer of D-(+)-Trehalose Anhydrous (CAS: 99-20-7) with high quality, production capacity 1500 tons per year. Ruifu Chemical has been supplying Trehalose for over 15 years. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase D-(+)-Trehalose Anhydrous, Please contact: alvin@ruifuchem.com
| Chemical Name | D-(+)-Trehalose Anhydrous |
| Synonyms | D-(+)-Trehalose; D-Trehalose; Trehalose; α,α-Trehalose Anhydrous; alpha-D-Trehalose; α-D-Glucopyranosyl-α-D-Glucopyranoside Anhydrous |
| Stock Status | In Stock, Production Capacity 1500 Tons per Year |
| CAS Number | 99-20-7 |
| Molecular Formula | C12H22O11 |
| Molecular Weight | 342.30 g/mol |
| Melting Point | 203℃ |
| Density | 1.76g/cm3 |
| Store Under Inert Gas | Store Under Inert Gas |
| Sensitive | Hygroscopic |
| Solubility | Easily Soluble in Water, Insoluble in Ether |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories |
Carbohydrates Sugars |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White Crystals or Crystalline Powder | White Crystalline Powder |
| Solubility | Easily Soluble in Water, Insoluble in Ether | Complies |
| Color and Clarity of Solution | Solubility in H2O Clear | Complies |
| Specific Rotation [a]20/D | +197.0° ~ +201.0° (C=7 in H2O) | +199.1° |
| Melting Point | 200.0~208.0℃ | 203.0~205.0℃ |
| pH | 5.0~6.7 | 6.21 |
| Loss on Drying | ≤1.00% | 0.08% |
| Residue on Ignition | ≤0.10% | 0.04% |
| Related Substances | ≤0.50% | <0.50% |
| Heavy Metals (Pb) | ≤5ppm | <5ppm |
| Arsenic (As) | ≤0.50 mg/kg | <0.33 mg/kg |
| Lead (Pb) | ≤0.50 mg/kg | <0.50 mg/kg |
| Assay / Analysis Method | >99.0% (HPLC) | 99.31% |
| Total Aerobic Microbial Count | ≤100cfu/g | <10cfu/g |
| Yeasts and Moulds | ≤100cfu/g | <10cfu/g |
| Salmonella | Negative | Negative |
| E. Coli | Negative | Negative |
| Staphylococcus | Negative | Negative |
| Infrared Spectrum | Consistent with Structure | Complies |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed, store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by sea, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xi - Irritant
Risk Codes 38 - Irritating to the skin
Safety Description S37/39 - Wear suitable gloves and eye/face protection
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
RTECS LZ5776770
TSCA Yes
HS Code 2940009090
D-(+)-Trehalose Anhydrous (CAS: 99-20-7) is used in cosmetics, foods, and parenteral and nonparenteral pharmaceutical formulations, agriculture and biotechnology. It is generally regarded as a relatively nontoxic and nonirritant material when used as an excipient. D-(+)-Trehalose Anhydrous can be used as a food ingredient and pharmaceutical excipient.
D-(+)-Trehalose Anhydrous is used as a food additive and sweetener. D-(+)-Trehalose Anhydrous has about 45% the sweetness of sucrose at concentrations above 22%. It can reduce the sweetness, optimize the sweet and improve the flavor.
Trehalose is also known for its ability to stabilize proteins and protect them from denaturation.
1. Mild sweetness
Trehalose is only 45% as sweet as sucrose. It has a clean taste profile
2. Process stability
Trehalose is a non-reducing sugar and will not take part in Mallard reactions with amino acids or proteins during the manufacturing processing and storage even at elevated temperatures. Thus, trehalose can be favorably used in food and beverage where heat treatment is required or storage at high temperature.
3. Perfect heat and acid tolerance
Trehalose is very stable to heat and acid during processing and storage, and would not become colored and broken down even heated for 30min at 100, pH3.0.
4. Low solubility and excellent crystalline
Water-solubility of trehalose is as high as maltose while the crystallinity is excellent, so it is easy to produce the low hygroscopical candy, coating, soft confectionery etc.
5. Low hygroscopicity Application
Some foods themselves are not hygroscopic, but their hygroscopicity can rapidly increase once sugars such as sucrose and maltose is added, and then foods' original flavor and shelf life changed. Trehalose has a very low hygriscopicity and remains stable up to 95% relative humidity. Thus, trehalose can be used as sweetening in food process.
In The Food & Healthcare Industry:
Trehalose are widely be used in food industry, such as Chocolate, candy, drinks, baking products,
frozen food, frozen seafish etc....
1. Low hygroscopicity, strong moisture holding capability, inhibit starch aging,enhance anti-freeze capability.
2. Heat resistance, acid resistance, improved freezing performance, trehalose is the most stable of natural disaccharide.
3. No colorability, trehalose does not cause the maillard reaction.
4. High glass transition temperature, high stability, prevent dental caries.
5. Prevent protein denaturation and lipid oxidation, protect biological activity.
6. Modify or cover the taste, improve, remove or enhance foods' special smell and taste.
7. Lasting and stable energy source, does not cause rapid and sharp change of blood sugar.
In The Cosmetics Industry:
Trehalose can be used as Moisturizes agent or smoothes agent, it has functions of Moisturizes and smoothes
to the skin and hair from dehydration under dry conditions.
1. Strongly moisturize capacity, keep skin epithelial cells moist even in extreme environments.
2. Anti-radiation.
3. Protect active ingredients in cosmetics.
4. Inhibit the characteristic body odor of the elderly.
5. Enhance skin's absorption ability to nutrition ingredients.
6. Prolong cosmetics' shelf life.
7. Prevent dental caries.
In The Agriculture Industry:
Enhance the anti-adversity ability of plant seeds, prolong the flowering period of fresh flower.
Trehalose
C12H22O11 342.30
C12H22O11·2H2O 378.33
α-D-Glucopyranosyl α-D-glucopyranoside.
Anhydrous [99-20-7].
Dihydrate [6138-23-4].
DEFINITION
Trehalose is a stable, nonreducing disaccharide with two glucose molecules linked in an α,α-1,1 configuration. It is obtained through enzymatic conversion of food-grade starch. It contains NLT 97.0% and NMT 102.0% of C12H22O11, calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption <197K>
• B.
Sample solution: 400 mg/mL of Trehalose
Analysis: Add 0.4 mL of a solution containing 1-naphthol in 95% alcohol (1 in 20) to 1 mL of the Sample solution. Gently add 2 mL of sulfuric acid to the solution.
Acceptance criteria: A violet color develops at the interface between the two solutions.
• C.
Glycine solution: 40 mg/mL of Glycine
Sample solution: 40 mg/mL of Trehalose
Analysis: Add 1 mL of diluted hydrochloric acid to 2 mL of the Sample solution. Allow to stand for 20 min at room temperature. Add 4 mL of sodium hydroxide TS and 2 mL of the Glycine solution to the Sample solution. Heat the solution for 10 min in boiling water.
Acceptance criteria: A brown color does not develop.
ASSAY
• Procedure
Mobile phase: Water
Standard solution: 10 mg/mL of USP Trehalose RS, calculated on the anhydrous basis
Sample solution: 10 mg/mL of Trehalose, calculated on the anhydrous basis
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: Refractive index
Column: 8-mm × 30-cm; packing L58
Temperature
Detector: 40
Column: 80
Flow rate: Adjust so that the retention time of trehalose is about 15 min.
Injection size: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of trehalose (C12H22O11) in the portion of Trehalose taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of the Sample solution
rS = peak response of the Standard solution
CS = concentration of USP Trehalose RS in the Standard solution (mg/mL)
CU = concentration of Trehalose in the Sample solution (mg/mL)
Acceptance criteria: 97.0%-102.0% on the anhydrous basis
IMPURITIES
• Residue on Ignition <281>: NMT 0.1%, determined on 2.0 g of Trehalose
• Heavy Metals, Method I <231>
Sample: 4.0 g
Monitor preparation: Prepare with 2.5 mL of Standard Lead Solution.
Acceptance criteria: NMT 5 ppm
• Related Substances
Mobile phase and Chromatographic system: Prepare as directed in the Assay.
Sample solution: 10 mg/mL of Trehalose
Standard solution: 0.1 mg/mL of Sample solution
System suitability solution: Dissolve 2.5 mL of Sample solution, 25 mg of maltotriose, and 25 mg of glucose, and dilute with water to 10.0 mL.
System suitability
Sample: System suitability solution
[Note-The relative retention times for maltotriose, trehalose, and glucose are about 0.9, 1.0, and 1.2, respectively.]
Suitability requirements
Resolution: NLT 1.5 between trehalose and maltotriose
Relative standard deviation: NMT 2.0% for the trehalose peak
Analysis
Samples: Sample solution and Standard solution
Determine the peak areas for all peaks.
Acceptance criteria: For the Sample solution, the areas of any peaks corresponding to maltotriose and other polysaccharrides and eluting before trehalose are NMT half of the area of the peak corresponding to trehalose in the chromatogram of the Standard solution (0.5%). The areas of any peaks corresponding to glucose and eluting after trehalose are NMT half of the area of the peak corresponding to trehalose in the chromatogram of the Standard solution (0.5%).
SPECIFIC TESTS
• Color and Clarity of Solution
Sample solution: 33 g of Trehalose in 67 g of recently boiled water
Analysis
Using a suitable spectrophotometer (see Spectrophotometry and Light-Scattering 851), measure the absorbances of the Sample solution at 420 and 720 nm in a 10-cm cuvette. The absorbance of the Sample solution at 720 nm is NMT 0.050.
Determine the absorbance difference:
Result = A420 A720
A420 = absorbance of the Sample solution at 420 nm
A720 = absorbance of the Sample solution at 720 nm
Acceptance criteria: The absorbance difference is NMT 0.100.
• Optical Rotation, Specific Rotation <781S>: +197° to +201° at 20
Sample solution: 100 mg/mL
• Microbial Enumeration Tests <61> and Tests for Specified Microorganisms <62>: The total aerobic microbial count is NMT 100 cfu/g, and the total combined molds and yeasts count is NMT 100 cfu/g. It meets the requirements of the tests for absence of Salmonella species and Escherichia coli.
• pH <791>
Sample solution: 100 mg/mL
Acceptance criteria: 4.5-6.5
• Water Determination, Method I <921>: For the anhydrous form, NMT 1.0%; for the dihydrate form, 9.0%-11.0%
Sample: 0.1g
• Bacterial Endotoxins Test <85>: If labeled for use in preparing parenteral dosage forms, it also meets the following requirements. The level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Trehalose is used can be met. Where the label states that Trehalose must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Trehalose is used can be met.
• Chloride and Sulfate, Chloride <221>: A 2.0-g sample shows no more chloride than corresponds to 0.70 mL of 0.01 M hydrochloric acid (NMT 0.0125%).
• Chloride and Sulfate, Sulfate <221>: A 2.0-g sample shows no more sulfate than corresponds to 0.83 mL of 0.005 M sulfuric acid (NMT 0.0200%).
• Nitrogen Content, Method I <461>
Sample: 5.0 g
Analysis: Proceed as directed for Method I, increasing the sulfuric acid used for digestion to 30 mL and reducing the sodium hydroxide solution (2 in 5) to 45 mL.
Acceptance criteria: NMT 0.005%
• Soluble Starch
Sample solution: 10% Trehalose (w/v)
Analysis: Add several drops of iodine TS to the Sample solution.
Acceptance criteria: No blue color develops.
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in tight containers. No storage requirements specified.
• Labeling: Where Trehalose is intended for use in the manufacture of injectable dosage forms, it is so labeled. Where Trehalose must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of bacterial endotoxins, it is so labeled.
• USP Reference Standards <11>
USP Endotoxin RS
USP Glycerin RS
USP Trehalose RS
-
D-(+)-Trehalose Anhydrous CAS 99-20-7 Assay >99...
-
D-(+)-Trehalose Dihydrate CAS 6138-23-4 Assay >...
-
Sucralose CAS 56038-13-2 Assay 98.0~102.0% Factory
-
D-(+)-Maltose Monohydrate CAS 6363-53-7 Assay ≥...
-
Lactulose CAS 4618-18-2 Assay >98.5% (HPLC)
-
Dulcitol (Galactitol) CAS 608-66-2 Assay ≥99.5%...
-
D-(+)-Cellobiose CAS 528-50-7 Assay >98.0% (HPLC)
-
D-Tagatose CAS 87-81-0 Assay >99.0% (HPLC) Factory
-
D-Glucal CAS 13265-84-4 Assay >96.0% (HPLC) Fac...
-
D-(+)-Glucose Anhydrous CAS 50-99-7 Assay ≥99.5...
-
D-Mannosamine Hydrochloride CAS 5505-63-5 Assay...
-
D-(+)-Galactose Anhydrous CAS 59-23-4 Assay >98...
-
D-(+)-Glucosamine Hydrochloride CAS 66-84-2 Ass...
-
D-(+)-Raffinose Pentahydrate CAS 17629-30-0 Ass...
-
D-(+)-Mannose CAS 3458-28-4 Assay >99.0% (HPLC)...
-
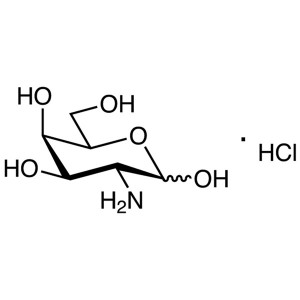
D-(+)-Galactosamine Hydrochloride CAS 1772-03-8...